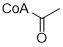
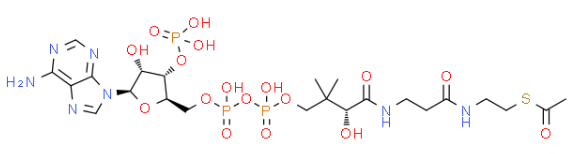
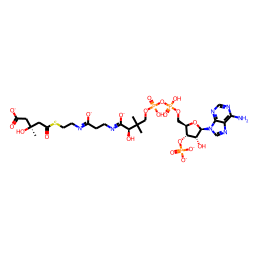
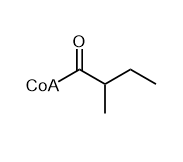
1.De novo biosynthesis of alpha-zingiberene from glucose in Escherichia coli. Zhang, Suping et al. Biochemical Engineering Journal, 176, 108188; 2021
2.Engineering potassium activation into biosynthetic thiolase. Marshall, Andrew C. and Bruning, John B.. Biochemical Journal, 478(15), 3047-3062; 2021
3.Identification of an α-Oxoamine Synthase and a One-Pot Two-Step Enzymatic Synthesis of α-Amino Ketones. Zhou, Ting et al. Organic Letters, 23(1), 37-41; 2021
4.Engineered ethanol-driven biosynthetic system for improving production of acetyl-CoA derived drugs in Crabtree-negative yeast. Liu, Yiqi et al. Metabolic Engineering, 54, 275-284; 2019
5.Metabolic engineering of Clostridium acetobutylicum for the production of butyl butyrate. Noh, Hyeon Ji et al. Applied Microbiology and Biotechnology, 102(19), 8319-8327; 2018
6.Biocatalytic Total Synthesis of Ikarugamycin. Greunke, Christian et al. Angewandte Chemie, International Edition, 56(15), 4351-4355; 2017
7.Chemoenzymatic Synthesis of Acyl Coenzyme A Substrates Enables in Situ Labeling of Small Molecules and Proteins. Agarwal, Vinayak et al. Organic Letters, 17(18), 4452-4455; 2015
8.Synthesis of coenzyme A thioesters using methyl acyl phosphates in an aqueous medium. Pal, Mohan and Bearne, Stephen L.. Organic & Biomolecular Chemistry, 12(48), 9760-9763; 2014
9.LsrF, a coenzyme A-dependent thiolase, catalyzes the terminal step in processing the quorum sensing signal autoinducer-2. Marques, Joao C. et al. Proceedings of the National Academy of Sciences of the United States of America, 111(39), 14235-14240; 2014
10.Extending Carbon Chain Length of 1-Butanol Pathway for 1-Hexanol Synthesis from Glucose by Engineered Escherichia coli. Dekishima, Yasumasa et al. Journal of the American Chemical Society, 133(30), 11399-11401; 2011
11.Substrate Specificity of 2-Hydroxyglutaryl-CoA Dehydratase from Clostridium symbiosum: Toward a Bio-Based Production of Adipic Acid. Parthasarathy, Anutthaman et al. Biochemistry, 50(17), 3540-3550; 2011
12.On the thermodynamic equilibrium between (R)-2-hydroxyacyl-CoA and 2-enoyl-CoA. Parthasarathy, Anutthaman et al. FEBS Journal, 277(7), 1738-1746; 2010
13.Point Mutations (Q19P and N23K) Increase the Operational Solubility of a 2α-O-Benzoyltransferase that Conveys Various Acyl Groups from CoA to a Taxane Acceptor. Nawarathne, Irosha N. and Walker, Kevin D.. Journal of Natural Products, 73(2), 151-159; 2010
14.An N-Aroyltransferase of the BAHD Superfamily Has Broad Aroyl CoA Specificity in Vitro with Analogues of N-Dearoylpaclitaxel. Nevarez, Danielle M. et al. Journal of the American Chemical Society, 131(16), 5994-6002; 2009
15.Thioester hydrolysis and C-C bond formation by carboxymethylproline synthase from the crotonase superfamily. Batchelar, Edward T. et al. Angewandte Chemie, International Edition, 47(48), 9322-9325; 2008
16.Bacillus cereus strain 10-L-2 produces two arylamine N-acetyltransferases that transform 4-phenylenediamine into 4-aminoacetanilide. Mulyono et al. Journal of Bioscience and Bioengineering, 103(2), 147-154; 2007
17.A simple and efficient method to prepare thio-esters in aqueous solutions. Coleman, Tricia M. et al. Tetrahedron Letters, 46(25), 4307-4310; 2005
18.6-Deoxyerythronolide B Analogue Production in Escherichia coli through Metabolic Pathway Engineering. Kennedy, Jonathan et al. Biochemistry, 42(48), 14342-14348; 2003